Need Help? Call us at: +91 9002 1002 33 (9:00 AM - 5:00 PM)
Email us: info@altuslifecares.com
No products in the cart.
Return To ShopNo products in the cart.
Return To Shop$409.09 – $1,850.65Price range: $409.09 through $1,850.65
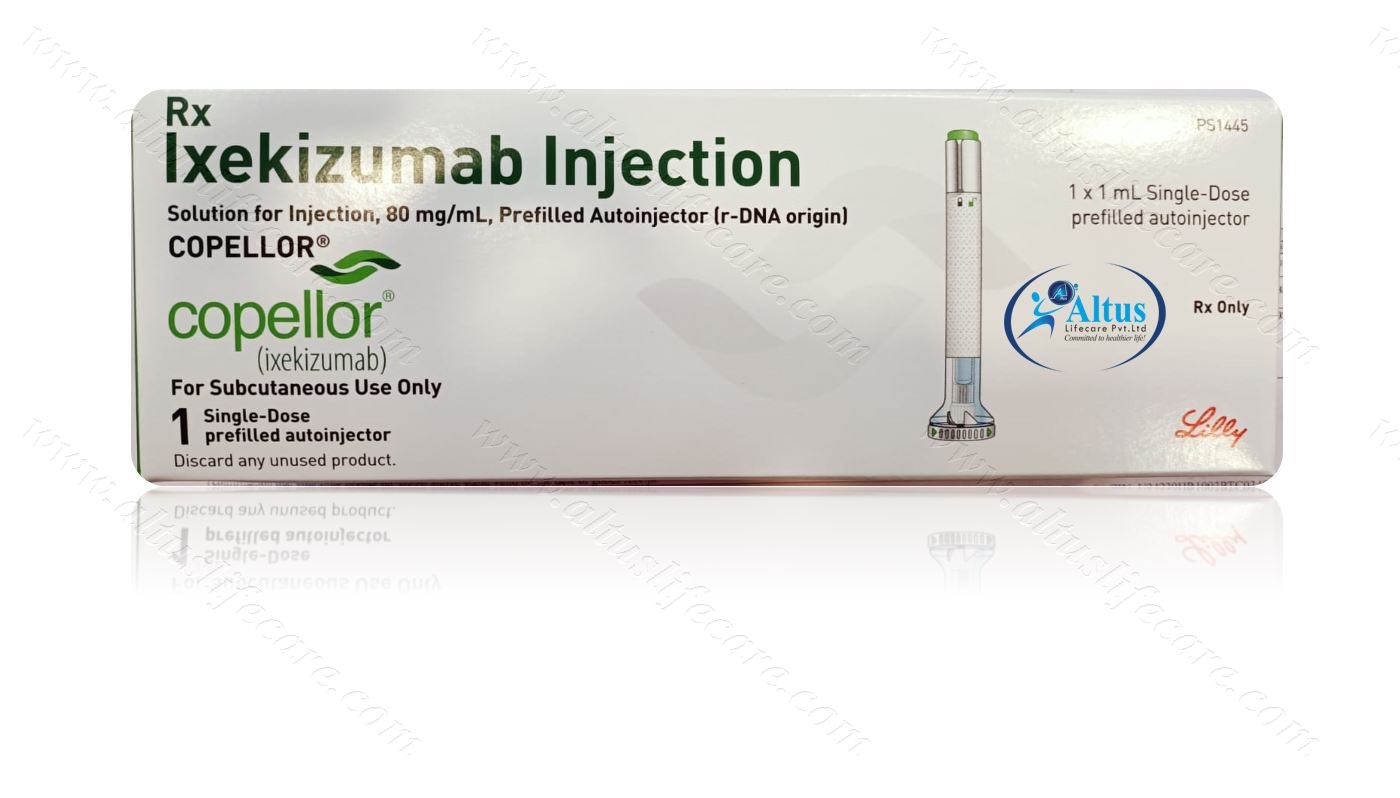
Copellor Ixekizumab Injection Psoriasis is a chronic auto-immune disorder of the skin in which prolonged inflammation leads to dry, thick, raised, red patches on the skin that continuously shed silvery scales.
Call : +91 9002 1002 33
| COUNTRY OF ORIGIN | India |
|---|---|
| DOSAGE FORM | Injection |
| GENERIC NAME | Ixekizumab Injection |
| INDICATION | Treatment of plaque psoriasis |
| PACKAGING | 80 mg/ml in a single dose prefilled auto-injector |
| MANUFACTURER | Eli Lilly |
| COMPOSITION | Ixekizumab Injection |
Copellor Ixekizumab Injection is used to treat moderate to severe plaque psoriasis (a skin disease in which red, scaly patches form on some areas of the body) in adults and children 6 years of age and older whose psoriasis is too severe to be treated by topical medications alone. It is also used alone or in combination with certain medications such as methotrexate (Rasuvo, Trexall, others) to treat psoriatic arthritis (a condition that causes joint pain and swelling and scales on the skin) in adults. Ixekizumab injection is also used to treat ankylosing spondylitis (a condition in which the body attacks the joints of the spine and other areas, causing pain and joint damage) in adults. It is also used to treat active non-radiographic axial spondyloarthritis (a condition in which the body attacks the joints of the spine and other areas causing pain and signs of swelling, but without changes seen on x-ray) in adults, Ixekizumab injection is in a class of medications called monoclonal antibodies. It works by blocking the action of a certain natural substance in the body that causes the symptoms of psoriasis.
Ixekizumab injection comes as a solution (liquid) in a prefilled syringe and as a prefilled autoinjector to inject subcutaneously (under the skin). To treat plaque psoriasis in adults, it is usually given as two injections for the first dose, followed by one injection every 2 weeks for the next 6 doses, and then one injection every 4 weeks. To treat plaque psoriasis in children, it is usually given as one or two injections for the first dose, depending on the weight of the child, followed by one injection every 4 weeks. To treat psoriatic arthritis or ankylosing spondylitis, it is usually given as two injections for the first dose, followed by one injection every 4 weeks. To treat non-radiographic axial spondyloarthritis, it is usually given as one injection every 4 weeks. You may receive your first dose of ixekizumab injection in your doctor’s office. If you are an adult, your doctor may allow you or a caregiver to perform the ixekizumab injections at home after your first dose. If you have vision or hearing problems, you will need a caregiver to give you injections. If your child weighs 110 pounds (50 kg) or less, ixekizumab injection must be given in a doctor’s office. If your child weighs more than 110 pounds, your doctor may allow a caregiver to perform the injections at home. Ask your doctor or pharmacist to show you or the person who will be injecting the medication how to inject and prepare it. Use each syringe or autoinjector only once and inject all the solution in the syringe or autoinjector. Dispose of used syringes and autoinjector in a puncture-resistant container. Talk to your doctor or pharmacist about how to dispose of the puncture-resistant container. Remove the prefilled syringe or autoinjector from the refrigerator. Place it on a flat surface without removing the needle cap and allow it warm to room temperature for 30 minutes before you are ready to inject the medication. Do not try to warm the medication by heating it in a microwave, placing it in hot water, leaving it in sunlight, or through any other method. Do not shake a syringe or autoinjector that contains ixekizumab.
Always look at ixekizumab solution before injecting it. Check that the expiration date has not passed and that the liquid is clear or slightly yellow. The liquid should not contain visible particles. Do not use a syringe or autoinjector if it is cracked or broken, if it is expired or frozen, or if the liquid is cloudy or contains small particles. You can inject ixekizumab injection anywhere on the front of your thighs (upper leg) or abdomen (stomach) except your navel and the area 1 inch (2.5 centimeters) around it. If you have a caregiver to inject the medication, the back of the upper arm may also be used. To reduce the chances of soreness or redness, use a different site for each injection. Do not inject into an area where the skin is tender, bruised, red, or hard or where you have scars or stretch marks. Do not inject ixekizumab into an area affected by psoriasis. Your doctor or pharmacist will give you the manufacturer’s patient information sheet (Medication Guide and Instructions for Use) when you begin treatment with ixekizumab injection and each time you refill your prescription. Read the information carefully and ask your doctor or pharmacist if you have any questions. You can also visit the Food and Drug Administration (FDA) website to obtain the Medication Guide, or the manufacturer’s website to obtain the Medication Guide and the Instructions for Use.
According to the Indianapolis-based company, Copellor will be a prescription medication that should only be used under medical supervision and upon the recommendation of a dermatologist or rheumatologist.
US pharma major Eli Lily on March 14 announced their foray into dermatology portfolio in India by launching the drug, Copellor Ixekizumab Injection, for the treatment of adults with moderate-to-severe plaque psoriasis and for the treatment of psoriatic arthritis.
The Indianapolis headquartered company said it recently received approval from The Drug Controller General of India (DCGI) for the launch of Copellor Ixekizumab Injection in India.
“Our foray into the dermatology segment strengthens Lilly’s promise of bringing innovative medicines to India. Global Studies show that the impact psoriasis has on a patient’s quality of life is comparable to that of ‘serious’ diseases such as cancer and heart failure,” Vineet Gupta, Managing Director, Eli Lilly, and Company – India & India Subcontinent said.
Gupta said the availability of a new treatment like Copellor Ixekizumab Injection will now empower healthcare providers with another option for the treatment of adults with moderate-to-severe plaque psoriasis, which was a huge unmet need in the country.
Eli Lily said Copellor will be a prescription medicine to be used only on the advice of a dermatologist or rheumatologist and under medical supervision.
RELATED STORIES

“Copellor Ixekizumab Injection is available in one strength of 80 mg/ml in a single dose prefilled auto-injector,” the company said.
Psoriasis is a chronic auto-immune disorder of the skin in which prolonged inflammation leads to dry, thick, raised, red patches on the skin that continuously shed silvery scales.
The company said these patches cause great discomfort to the patient because they are accompanied by unstoppable itching. Copellor Ixekizumab Injection can affect daily activities such as dressing, walking, cooking, and typing.
Several studies show that patients with severe psoriasis are prone to depression and may even develop a suicidal tendency.
Psoriasis can also lead to a painful condition of the joints known as ‘psoriatic arthritis.’
The research shows that in psoriasis, inflammation is present throughout the body and this increases the risk of heart disease, diabetes, kidney diseases, and inflammatory bowel diseases along with psoriasis.
There is no single cause identified for this condition, but there are a number of contributing factors: genetic risk, environmental triggers such as obesity, stress, some drugs, and injury.
| Pack Size | 1 Injection/s, 3 Injection/s, 5 Injection/s |
|---|

Benoquin 40 Cream | Monobenzone 40%


Aziderm 10% Cream 15gm | Azelaic Acid 10%
Shipping at Discounted Price
Return Within 30 Days
Safe & Secure Payment
Contact 24 Hours Day

From: $298.70
You cannot copy content of this page.
Taytum (verified owner) –
“The quality is outstanding, and the shipping was surprisingly fast!”