Ultravist 370mg Injection 100ml
| COUNTRY OF ORIGIN | India |
|---|---|
| DOSAGE FORM | Injection |
| GENERIC NAME | Iopromide |
| INDICATION | Diagnostic agent |
| PACKAGING: | 100 ml in 1 vial |
| MANUFACTURER | Zydus Cadila |
| COMPOSITION | Iopromide 370mg 100ml |
Description:
Ultravist 370mg Injection 100ml is a clear, colorless to pale yellow sterile injection containing iopromide 769 mg/mL, corresponding to 370 mg of iodine per milliliter. As a non-ionic, water-soluble radiographic contrast agent, it is used to enhance the visibility of internal body structures in a variety of X-ray and CT-based diagnostic procedures. The 100 mL volume presentation is designed for single-dose use in adult patients undergoing complex or multi-phase imaging studies that require larger contrast volumes.
Ultravist® 370 provides excellent image contrast due to its high iodine concentration while maintaining a favorable safety profile. Its low osmolality and non-ionic formulation make it well-tolerated and widely applicable in modern radiologic practice.
Indications:
Ultravist® 370 is indicated for use in a wide range of contrast-enhanced imaging studies, including:
✅ Computed Tomography (CT):
-
Enhancement of head, neck, chest, abdomen, and pelvic scans
-
Particularly useful in angiographic CT protocols (CTA)
-
Aids in the detection and characterization of tumors, vascular anomalies, and inflammatory conditions
✅ Angiography:
-
Cerebral angiography: Visualization of cerebral arteries and veins for stroke, AVMs, aneurysms
-
Coronary angiography: Used in assessing coronary artery disease
-
Peripheral angiography: Evaluation of limb ischemia or peripheral vascular disease
-
Visceral angiography: Imaging of abdominal vasculature including renal and hepatic arteries
✅ Intravenous Urography (IVU):
-
Imaging of renal pelvis, ureters, and bladder
-
Supports diagnosis of urinary tract obstruction, renal calculi, and congenital anomalies
✅ Venography:
-
Assessment of venous patency in cases of thrombosis or venous insufficiency
✅ Digital Subtraction Angiography (DSA):
-
High-precision imaging by digitally removing non-vascular structures
-
Particularly useful for neurovascular and interventional radiology
Key Features:
🔹 High Iodine Concentration (370 mg/mL) for Enhanced Diagnostic Clarity
Ultravist® 370 delivers one of the highest iodine concentrations available in non-ionic contrast media. This ensures optimal attenuation of X-rays, resulting in sharp and high-contrast images, even in challenging vascular territories or high-flow systems. Its use improves lesion detectability and vascular visualization, especially in cardiovascular and neurological imaging.
🔹 Low Osmolality for Increased Safety and Comfort
Ultravist® 370 features a low osmolality (~774 mOsm/kg H2O), which is significantly lower than that of older ionic contrast agents. This helps to reduce the incidence of adverse events, including vasodilation, pain, heat sensation, and other contrast-induced side effects. It is particularly beneficial in patients with cardiovascular comorbidities, diabetes, or those at risk for contrast-induced nephropathy (CIN).
🔹 Non-Ionic and Water-Soluble Formulation
Being non-ionic, Ultravist® does not dissociate into charged particles in solution. This minimizes chemotoxicity and the potential for triggering allergic-like reactions. Its high water solubility allows for rapid dilution and dispersion in blood, ensuring smooth intravascular administration and quick systemic distribution.
🔹 Rapid Renal Clearance for Minimal Systemic Retention
Ultravist® 370 is primarily eliminated unchanged by the kidneys. In patients with normal renal function, more than 90% of the injected dose is typically excreted within 24 hours. This reduces the duration of iodine exposure, minimizes systemic accumulation, and lowers the risk of delayed adverse effects, making it a safe choice for repeated or high-dose imaging protocols.
🔹 Versatility Across Imaging Modalities and Patient Groups
Ultravist® 370 is suitable for a wide range of imaging procedures across multiple disciplines, including radiology, cardiology, urology, and neurology. It is appropriate for use in both adult and pediatric populations (with adjusted dosing). The 100 mL size is ideal for high-volume requirements in multi-phase CT studies or extensive angiographic evaluations.
🔹 Clinically Proven Safety Profile
Ultravist® has been used in millions of procedures worldwide and is backed by extensive clinical data. Adverse events are typically mild and transient, including nausea, headache, or mild warmth at the injection site. Serious hypersensitivity reactions are rare. The product complies with international pharmacopoeia standards and undergoes stringent quality control processes to ensure reliability and safety.
Presentation: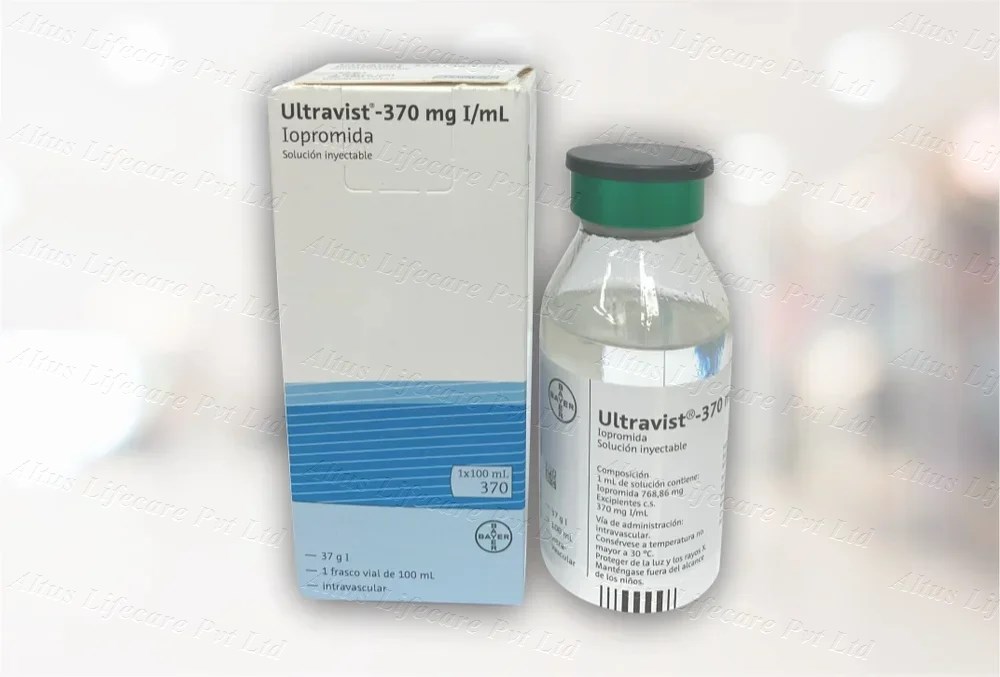
-
Supplied in a 100 mL glass vial or prefilled syringe
-
Single-use container
-
Clear, ready-to-use solution with no need for dilution or reconstitution
-
Packaged under sterile conditions, compliant with pharmaceutical-grade manufacturing practices
Storage Conditions:
-
Store below 30°C (86°F)
-
Do not freeze
-
Protect from light and keep in original packaging until use
-
Inspect the vial visually before administration—do not use if the solution is discolored, cloudy, or contains particulate matter
Caution & Administration Guidelines:
-
Ultravist® 370 should only be administered by qualified healthcare professionals trained in contrast media use and emergency management.
-
Assess renal function (e.g., serum creatinine or eGFR) prior to administration, especially in patients with pre-existing renal impairment or diabetes.
-
Maintain adequate hydration before and after administration to support renal clearance and reduce the risk of contrast-induced nephropathy.
-
Monitor the patient during and after the injection for any signs of hypersensitivity or side effects. Emergency resuscitation equipment should always be available during contrast-enhanced imaging procedures.
-
Use the lowest effective dose required to obtain diagnostic-quality images.











Reviews
There are no reviews yet.