No products in the cart.
Return To Shop
Menu
Categories
- Anti Fungal
- Hepatitis
- Beauty & Skin Care
- Asthma
- Modafinil
- Urology Segment
- Thyroid Care
- Armodafinil
- HIV Medicines
- Weight Loss
- Naltrexone
- Anti Emetic
- Neuropathic Pain
- Mens Health
- Hair Loss
- Pain Relief
- HCG Injections
- Quit Smoking
- Pharmaceutical Vaccine
- Best Selling Products
- Anti Viral
- Bimatoprost
- Antibiotics
- Women's Health
- Cetaphil
- Botulinum
- Diabetes
- Human Albumin
- Anti Malarial
- Dermal fillers
- Chemical Peels
- Nephrology Segment
- Kidney / Liver Care
- Anti Cancer
- Altus Product's
- Pharmaceutical Products
Wishlist
0
Sign in
Login
Register
- Anti-Cancer
- Armodafinil
- Bimatoprost
- Botulinum
- Dermal Fillers
- Hepatitis
- Mens-health
- Modafinil
- Naltrexone
- ANTI EMETIC
- Altus Product’s
- Anti Fungal
- Anti Malarial
- Anti Viral
- Antibiotics
- Asthma
- Beauty & Skin Care
- Cetaphil
- Chemical Peels
- Diabetes
- Hair Loss
- HCG Injections
- HIV Medicines
- Human Albumin
- Kidney / Liver Care
- Neuropathic Pain
- Pain Relief
- Pharmaceutical Products
- Pharmaceutical Vaccine
- Quit Smoking
- Thyroid Care
- Weight Loss
- Women’s Health
Shop and get discounts
Worldwide Shipping
Menu
Categories
- Anti Fungal
- Hepatitis
- Beauty & Skin Care
- Asthma
- Modafinil
- Urology Segment
- Thyroid Care
- Armodafinil
- HIV Medicines
- Weight Loss
- Naltrexone
- Anti Emetic
- Neuropathic Pain
- Mens Health
- Hair Loss
- Pain Relief
- HCG Injections
- Quit Smoking
- Pharmaceutical Vaccine
- Best Selling Products
- Anti Viral
- Bimatoprost
- Antibiotics
- Women's Health
- Cetaphil
- Botulinum
- Diabetes
- Human Albumin
- Anti Malarial
- Dermal fillers
- Chemical Peels
- Nephrology Segment
- Kidney / Liver Care
- Anti Cancer
- Altus Product's
- Pharmaceutical Products
Wishlist
0
0
Cart
$0.00
0
No products in the cart.
Return To Shop Shopping cart (0)
Subtotal: $0.00
Worldwide Shipping
Compare
Please, enable Compare.
SKU: N/A
Category: Pharmaceutical Products
$141.98 – $605.98Price range: $141.98 through $605.98
Have questions?
Our experts are ready to help.
Call : +91 9002 1002 33
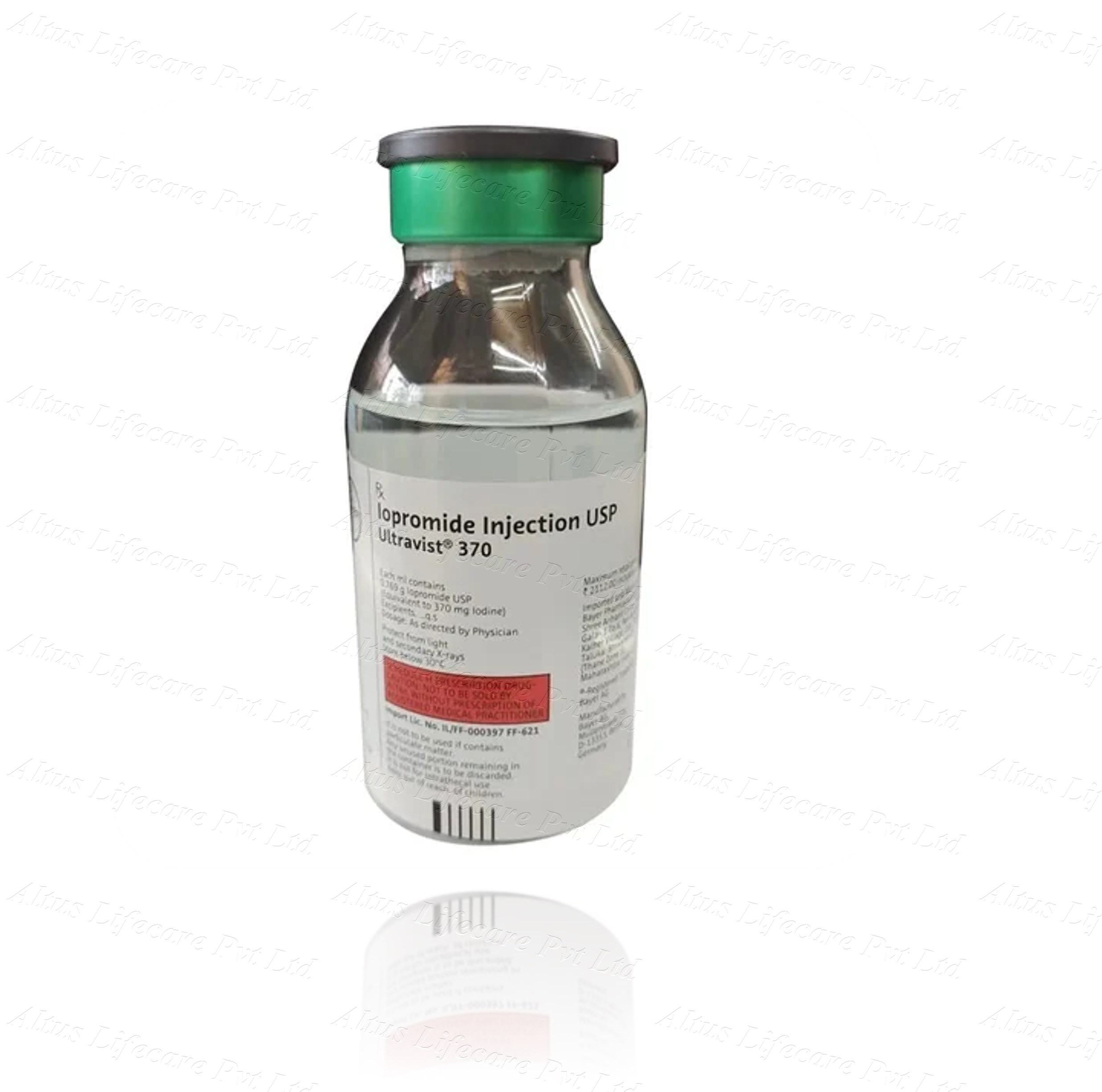
Ultravist 370mg Injection 50ml
| COUNTRY OF ORIGIN | India |
|---|---|
| DOSAGE FORM | Injection |
| GENERIC NAME | Iopromide |
| INDICATION | Diagnostic agent |
| PACKAGING: | 50 ml in 1 vial |
| MANUFACTURER | Zydus Cadila |
| COMPOSITION | Iopromide 370mg 50ml |
Description:
Ultravist 370mg Injection 50ml is a non-ionic, water-soluble contrast agent containing iopromide—a tri-iodinated, low-osmolar compound that provides high-contrast enhancement in radiographic imaging. Each milliliter of solution contains 769 mg of iopromide, delivering 370 mg of organically bound iodine. The formulation is designed for intravascular injection, enabling temporary opacification of vascular structures, organs, and body cavities to support accurate visualization during diagnostic procedures.
The 50 mL vial presentation is intended for single-use, offering a convenient and hygienic volume suitable for most routine and advanced diagnostic examinations. Ultravist® 370 is known for its excellent safety profile, ease of administration, and wide acceptance in both outpatient and inpatient imaging centers.
Indications:
Ultravist® 370 is indicated for use in a broad range of diagnostic imaging procedures requiring iodinated contrast enhancement:
-
Computed Tomography (CT):
Enhances contrast in CT scans of the head, chest, abdomen, pelvis, and spine, allowing for improved detection of lesions, tumors, vascular abnormalities, and organ pathology. -
Angiography:
Enables high-resolution imaging of the blood vessels, including:-
Coronary angiography – for assessment of coronary artery disease
-
Cerebral angiography – to detect aneurysms, AVMs, or stenosis
-
Peripheral and visceral angiography – for evaluation of limb ischemia, vessel malformations, or abdominal vasculature
-
-
Intravenous Urography (IVU):
Facilitates visualization of the kidneys, ureters, and bladder to detect urinary tract obstructions, renal calculi, and anatomical anomalies. -
Venography:
Used for imaging of the venous system, particularly in the diagnosis of deep vein thrombosis or venous insufficiency. -
Digital Subtraction Angiography (DSA):
Provides high-definition images of vascular structures by digitally removing bone and soft tissue, especially useful in neurological and cardiovascular imaging.
Key Features:
🔹 High Iodine Concentration for Superior Image Contrast
Ultravist® 370 delivers 370 mg of iodine per milliliter—one of the highest concentrations available—resulting in strong radiodensity and enhanced image clarity. This is particularly beneficial in high-speed CT imaging and detailed angiographic procedures where high contrast is crucial for detecting small lesions, vascular stenoses, or anomalies.
🔹 Low Osmolality for Enhanced Patient Safety
As a low-osmolar contrast medium (LOCM), Ultravist® 370 significantly reduces the risk of adverse events compared to older, high-osmolar agents. Its osmolality is closer to that of blood plasma, minimizing the potential for osmotic shifts that can cause discomfort, vasodilation, or endothelial damage. This makes it ideal for use in at-risk patients, such as the elderly, those with cardiovascular disease, or those prone to contrast reactions.
🔹 Non-Ionic, Water-Soluble Formulation
Ultravist® is non-ionic, meaning it does not dissociate into charged particles in solution, which contributes to its superior safety and tolerability. Its water solubility ensures smooth mixing in blood plasma and facilitates intravenous administration without causing precipitation or irritation. This formulation also supports clear imaging of both soft tissues and vascular structures with minimal artifact.
🔹 Rapid Elimination via Renal Excretion
Following administration, Ultravist® 370 is rapidly excreted unchanged through the kidneys. In individuals with normal renal function, more than 90% of the administered dose is typically cleared from the body within 24 hours. This rapid clearance reduces the potential for iodine accumulation and lowers the risk of nephrotoxicity, especially with appropriate hydration.
🔹 Flexible Use Across Modalities and Populations
Ultravist® 370 is approved for use across a wide range of imaging applications and is suitable for both adults and pediatric patients. Its versatility makes it a go-to contrast agent for radiologists, cardiologists, and interventional specialists. It is compatible with a variety of imaging technologies including spiral CT, multi-detector CT, and advanced angiographic systems.
🔹 Proven Clinical Safety Profile
Extensive clinical trials and decades of global use have established the safety and efficacy of Ultravist® 370. Adverse reactions are generally mild and infrequent, with hypersensitivity reactions occurring rarely. Its formulation has been refined for optimal tolerability, making it a trusted choice in both elective and emergency diagnostic settings.
Presentation:
-
Supplied in 50 mL single-use glass vials or prefilled syringes
-
Ready-to-use solution, no dilution or reconstitution required
-
Clear, colorless to pale yellow solution
-
Packaged in accordance with international safety and sterility standards
Storage Conditions:
-
Store below 30°C (86°F)
-
Do not freeze
-
Keep in original packaging to protect from light
-
Inspect visually prior to use—do not use if solution is discolored or contains particulate matter
Caution & Administration Guidelines:
-
Ultravist® 370 should be administered by qualified healthcare professionals with appropriate training in diagnostic imaging and contrast administration.
-
Assess renal function (e.g., serum creatinine or eGFR) before use, particularly in patients with risk factors for contrast-induced nephropathy.
-
Monitor for signs of allergic or hypersensitivity reactions during and after the procedure. Emergency equipment and medications should be readily available.
-
Ensure adequate hydration pre- and post-procedure to support renal clearance.
-
Use the smallest effective dose necessary to achieve the required diagnostic outcome.
| Pack Size | 5 Injection/s, 10 Injection/s, 15 Injection/s, 20 Injection/s, 30 Injection/s |
|---|
Be the first to review “Ultravist 370mg Injection 50ml” Cancel reply
Related products
Janumet 50mg-500mg Tablet
From: $110.00Lipicure 10 Tablet (Atorvastatin 10mg)
From: $33.83Alphadol Capsule | Alfacalcidol
From: $38.31Januvia 50mg Tablet (sitagliptin 50mg)
From: $63.90Eptus 50 Tablet (Eplerenone 50mg)
From: $66.23Istavel 50mg Tablet (Sitagliptin 50mg)
From: $39.22Buy Alfacal Capsule (Alfacalcidol 0.25mcg / 1mcg)
From: $38.96People also bought
-
Benoquin 40 Cream | Monobenzone 40%
From: $154.77 - From: $38.38
- From: $40.05
-
Aziderm 10% Cream 15gm | Azelaic Acid 10%
From: $39.26
Our Services
Shipping
Shipping at Discounted Price
Money Returns
Return Within 30 Days
Secure Payment
Safe & Secure Payment
Support 24/7
Contact 24 Hours Day
ULTRAVIST 300 Injection 100ml
From: $168.65


Ultravist 370mg Injection 100m...
From: $202.04
More
More
- Anti-Cancer
- Armodafinil
- Bimatoprost
- Botulinum
- Dermal Fillers
- Hepatitis
- Mens-health
- Modafinil
- Naltrexone
- ANTI EMETIC
- Altus Product’s
- Anti Fungal
- Anti Malarial
- Anti Viral
- Antibiotics
- Asthma
- Beauty & Skin Care
- Cetaphil
- Chemical Peels
- Diabetes
- Hair Loss
- HCG Injections
- HIV Medicines
- Human Albumin
- Kidney / Liver Care
- Neuropathic Pain
- Pain Relief
- Pharmaceutical Products
- Pharmaceutical Vaccine
- Quit Smoking
- Thyroid Care
- Weight Loss
- Women’s Health


















Reviews
There are no reviews yet.